INDICAID COVID-19 Rapid Antigen Test

The INDICAID COVID-19 Rapid Antigen Test is easy to use, and can be self-test anytime, anywhere to detect the presence of the SARS-CoV-2 virus.
- For in-vitro diagnostic use only.
About
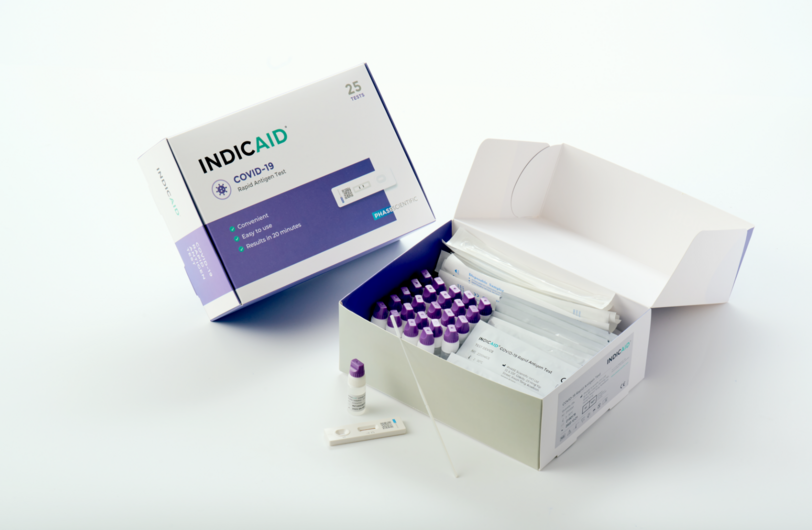
Features
- Hong Kong brand with locally developed technology
- Accurate result in 20 minutes
- Easy to use, no equipment or training needed
- Able to detect coronavirus variants (incl. Delta and Omicron)1
- Globally recognized, with authorization from US FDA EUA, EU CE certification and many other leading regulatory institutions around the world
Remarks
1 Able to detect coronavirus variants
Detectable variants include Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Delta Plus (AY.1 and AY.2), and Omicron (B.1.1.529, BA.2, XXB) (multiple variants)
2 U.S. FDA EUA
INDICAID®️ COVID-19 Rapid Antigen Test obtained U.S. FDA EUA in July 2021
Test Result Interpretation
| CT Indicator | Result | |
|---|---|---|
 | A line appears in regions (C) and (T) | Positive |
 | A line appears in region (C) | Negative |
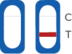 | No line appears in region (C) | Invalid |
Specifications
| Specimen type | Nasal and nasopharyngeal swab |
|---|---|
| Limit of Detection | 140 TCID50 / swab |
| Sensitivity | 96% |
| Specificity | 99% |
| Shelf life | 12 months |
| Storage condition | 2-30oC. Do not freeze. Avoid direct sunlight |
FAQs
An antigen is a molecule or molecular structure present in a pathogen that, when detected in the human body, can trigger an immune response. Common antigens include simple molecules such as carbohydrates, fats, hormones, and larger molecules including polysaccharides, phospholipids, nucleic acids, and proteins.
Antigens are present in SARS-CoV-2 viruses, which can often be found in the respiratory tract during early-stage infections (2-3 days). These antigens, when detected, can serve as markers for disease exposure.
The test uses nasal swab samples, which are collected from the nostrils.
Complete the test in just 4 simple steps:
- Collect anterior nasal swab specimen
- Stir the swab into the buffer solution
- Drop 3 drops of the buffer solution into the test device
- Read the results promptly in 20 minutes (results after 25 minutes should not be used)
The sample collection process is very simple. The swab only needs to be inserted about 1 inch into the nostrils (and not as far as the nasal cavity), which will not cause any pain or discomfort to the user.
Negative: A line appears in region (C)
Positive: A line appears in regions (C) and (T)
Invalid Result: No line appears in the region (C)
The accuracy of COVID-19 Antigen Tests is measured under two metrics.
- Sensitivity: Measures the ability to correctly identify positive patient samples. If a test kit has a high sensitivity, it means that the kit has a high positive percentage agreement and a low false negative rate
- Specificity: Measures the ability to correctly identify negative patient samples. If a test kit has a high specificity, it means that the kit has a high negative percentage agreement and a low false positive rate
INDICAID's overall sensitivity and specificity is 96% and 99% respectively, and is able to detect the presence of certain COVID-19 variants.
INDICAID has passed extensive clinical validation. In Hong Kong Government’s Community Testing Scheme, INDICAID™ has been used as part of the dual-track testing scheme along with COVID-19 PCR tests. As of January 2021, after the completion of 22994 clinical tests, it has been shown to reach accuracy comparable to PCR tests. This is the world's largest clinical study for a product of its kind.
COVID-19 is mainly airborne, and will first pass through the nasal cavity before entering the upper respiratory tract. Medical research shows a relatively high virus content can be found in nasal swab samples. Using nasal swab samples for COVID-19 testing is done internationally. Compared to other sampling methods, the use of nasal swab samples can guarantee a higher test sensitivity.
Product Resources
INDICAID Rapid Antigen Test Instruction Guide











