INDICAID COVID-19 Rapid Antibody Test
About
Features
- Accurate result in 10 minutes
- Easy to use, no equipment or training needed
- Globally recognized, with authorization from US FDA EUA and EU CE certification
Test Result Interpretation
| Indicator | Result | Interpretation | |
|---|---|---|---|
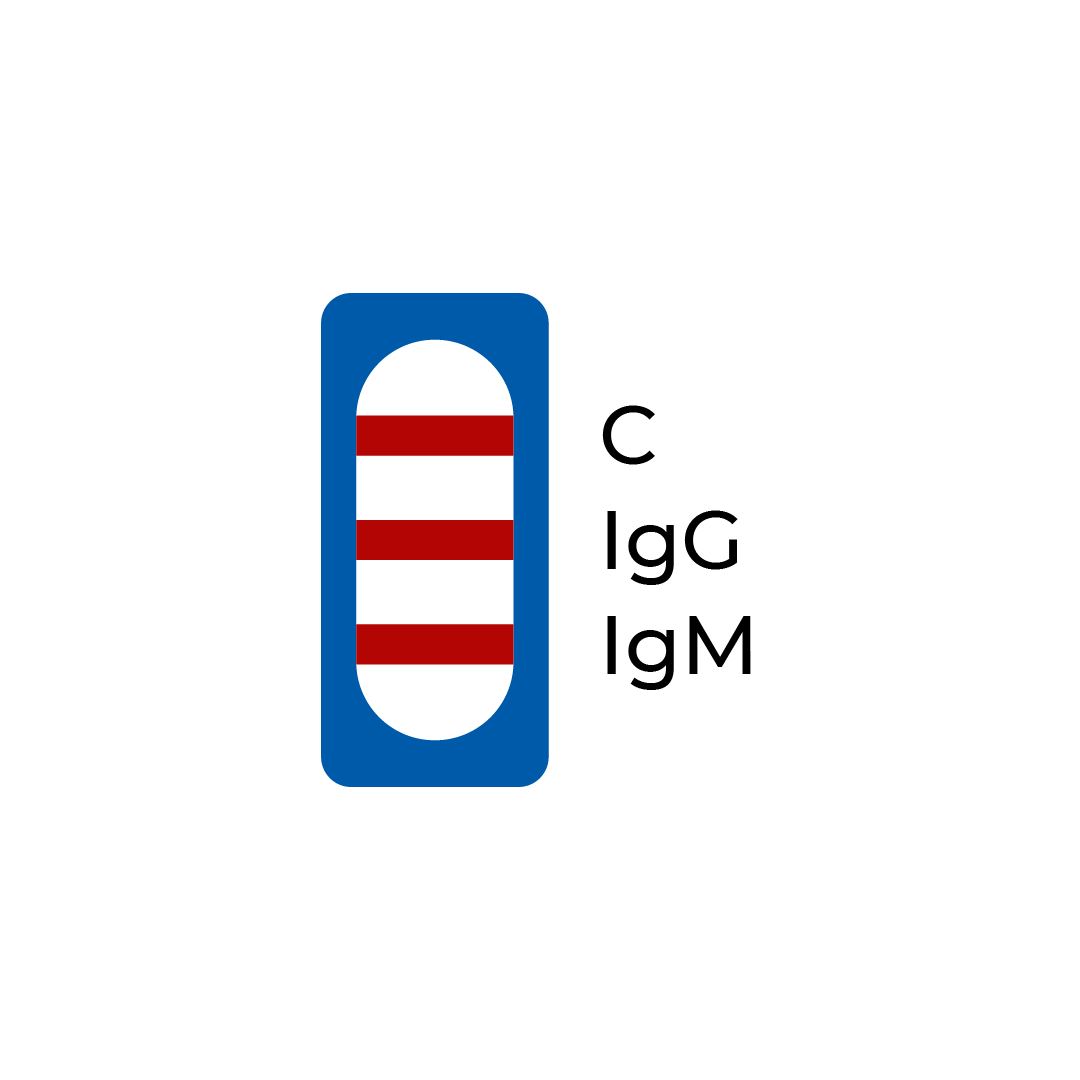 | A line appears in regions (C), (IgG) and (IgM) | Positive | The presence of the control line (C) and both the IgM line (IgM) and IgG line (IgG) indicates the presence of both SARS-CoV-2 IgM and IgG antibodies. The result suggests current or recent COVID-19 infection. |
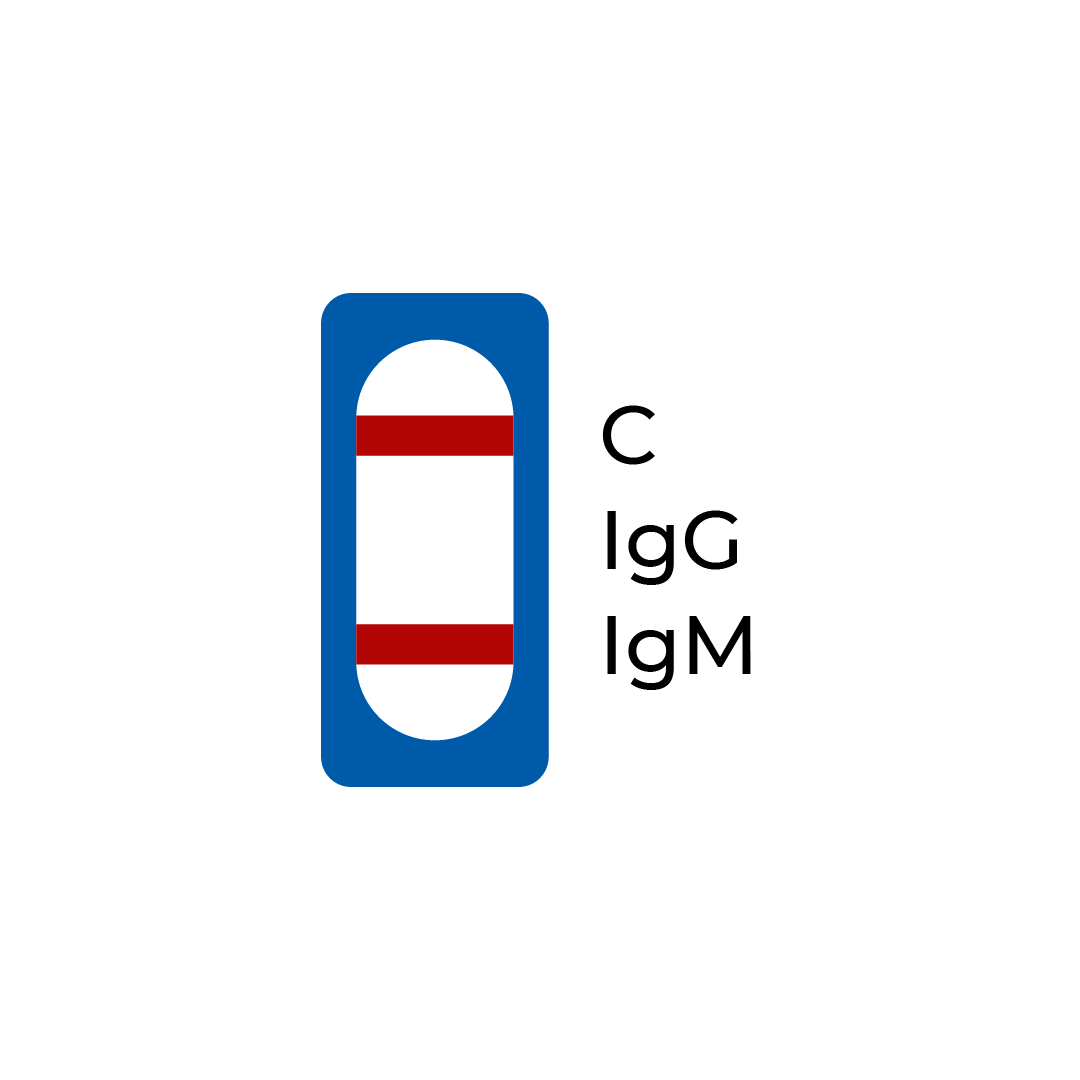 | A line appears in region (C) and (IgM) | Positive | The presence of the control line (C) and only the IgM line (IgM) indicates the presence of SARS-CoV-2 IgM antibodies. The result suggests an active or recent COVID-19 infection. |
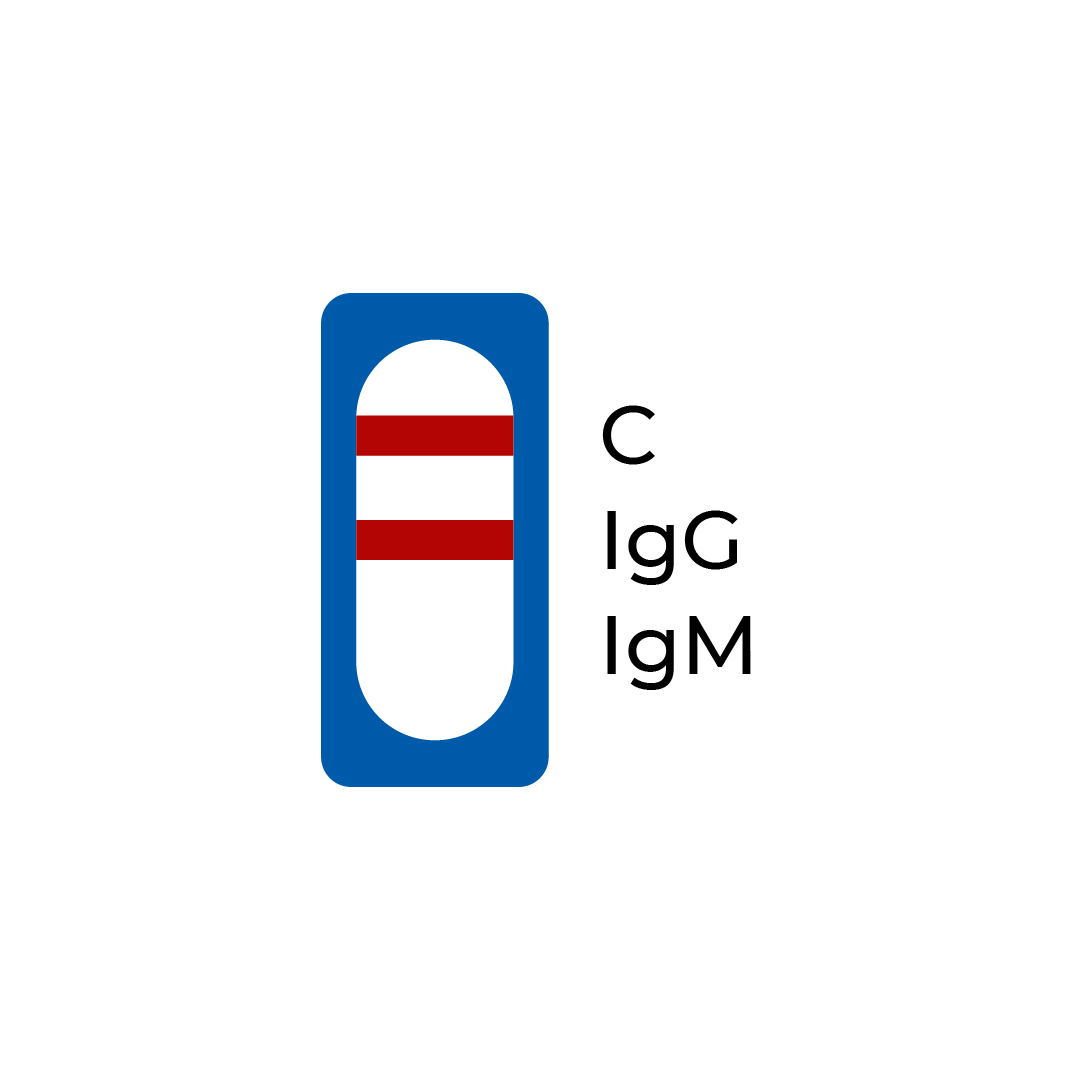 | A line appears in region (C) and (IgG) | Positive | The presence of the control line (C) and only the IgG line (IgG) indicates the presence of SARS-CoV-2 IgG antibodies. The result suggests a recent or previous COVID-19 infection. |
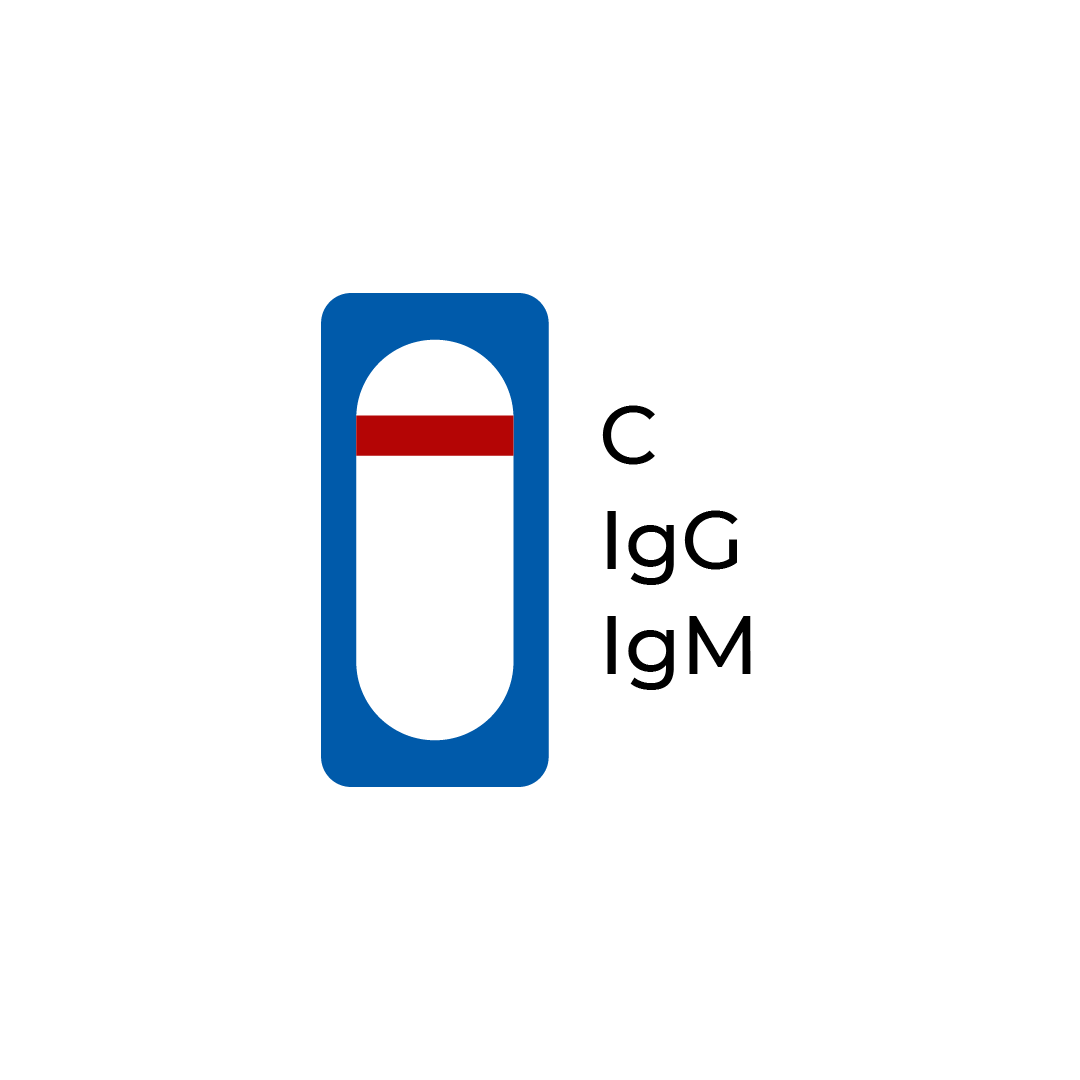 | A line appears in the region (C) | Negative | The presence of only the control line (C) and neither the IgM line (IgM) or the IgG line (IgG), indicates no detection of SARS-CoV-2 IgM or IgG antibodies. |
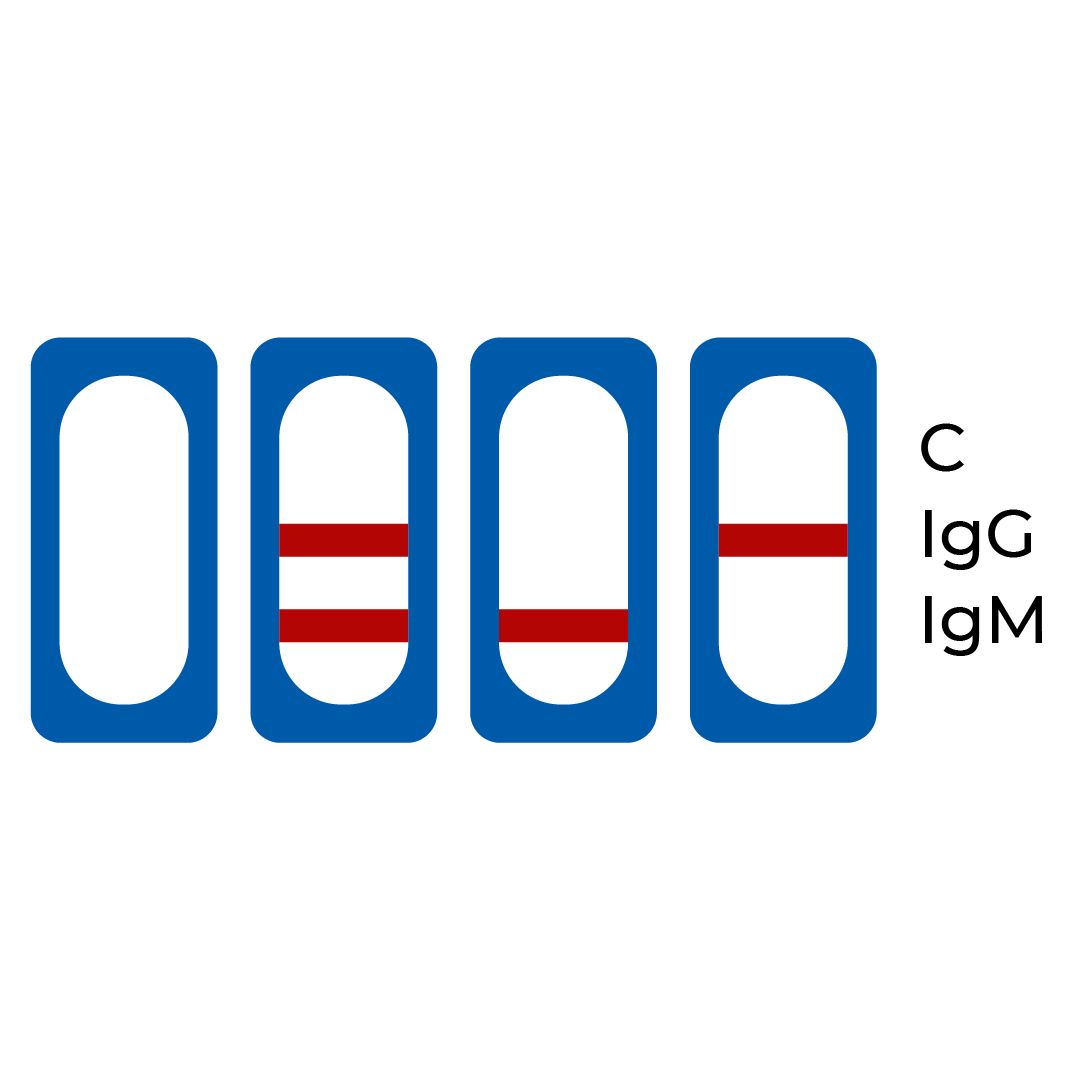 | No line appears in the region (C) | Invalid | If the control line (C) does not appear, the result is invalid, regardless whether the IgM line (IgM) or the IgG line (IgG) is present. Repeat the test with a new test kit. |
Specifications
| Specimen type | Human whole blood, serum, or plasma |
|---|---|
| IgM Sensitivity | 97.1% |
| IgM Specificity | 99% |
| IgG Sensitivity | 95.7% |
| IgG Specificity | 99% |
| Overall Sensitivity | 97.1% |
| Overall Specificity | 99% |
| Shelf life | 24 months |
| Storage condition | 2-30oC. Do not freeze. Avoid direct sunlight |
FAQs
As your body starts to fight off infection, the immune system is triggered by the presence of the antigens and begins to produce antibodies to counteract the virus. Antibodies that fight the SARS-CoV-2 virus includes immunoglobulin M (IgM), which is produced 5-7 days after infection, and immunoglobulin G (IgG), with is produced 10-15 days after infection.
The immune system begins to produce antibodies around 7 days after infection. Through detecting the presence of SARS-CoV-2-specific antibodies, the COVID-19 antibody test can determine the status of infection. COVID-19 antibody tests are used on blood or plasma samples, as antibodies are commonly found.
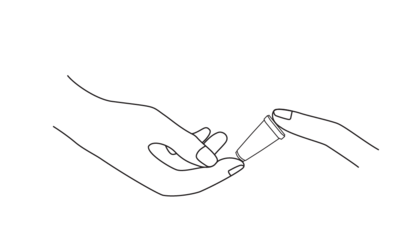
The test uses human whole blood collected venously or from fingerstick. It can also use serum or plasma spun down from human whole blood.
Complete the test in just 4 simple steps:
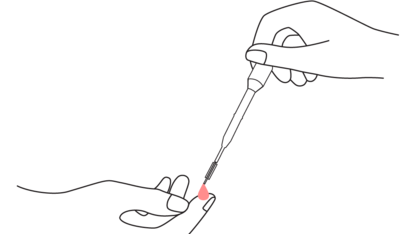
1. Collect whole blood specimen
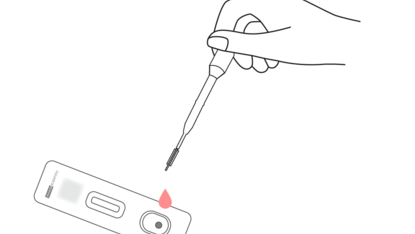
2. Drip all of the blood into the test device
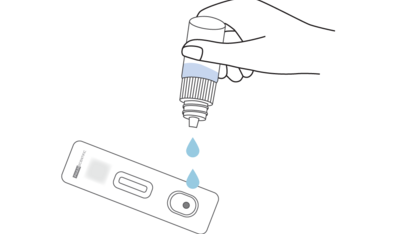
3. Drop 2 drops of the buffer solution into the test device
4. Read the results promptly in 10 minutes (results after 15 minutes should not be used)
After you have pricked your finger with the lancet, you can draw your blood using the pipette, and add a single drop to the sample well.
Negative: A line appears in region (C)
Positive: A line appears in either:
- region (C) and region (IgM),
- region (C) and region (IgG), or
- region (C), region (IgM), and region (IgG)
Invalid Result: No line appears in the region (C)
The accuracy of COVID-19 antibody Tests is measured under two metrics.
- Sensitivity: Measures the ability to correctly identify positive patient samples. If a test kit has a high sensitivity, it means that the kit has a high positive percentage agreement and a low false negative rate
- Specificity: Measures the ability to correctly identify negative patient samples. If a test kit has a high specificity, it means that the kit has a high negative percentage agreement and a low false positive rate
INDICAID™'s overall sensitivity and specificity is 97.1% and 99% respectively.
INDICAID has passed an independent clinical validation. In a clinical trial conducted by a top Hong Kong university, 50 positive and 50 negative patient samples previously confirmed with RT-PCR were independently tested using the INDICAID™ COVID-19 Rapid Antibody Test Kit. The results indicated an overall sensitivity and specificity of 96%and 96% respectively.
If you are not vaccinated and have gotten a positive result from the INDICAID COVID-19 IgM/IgG Rapid Test, it is likely that you are currently or previously infected with SARS-CoV-2. Please contact your medical provider or local health authorities immediately to arrange a COVID-19 PCR test for diagnosis.
INDICAID COVID-19 Rapid Antibody Test can only determine whether antibodies are present in the blood at the time of test. During the early stage of an infection, it is possible to receive a negative test result due to the body producing antibodies at a concentration level below the limit of detection of the test. Hence, this product cannot rule out the possibility of SARS-CoV-2 infection. If you have been in contact with a suspected or confirmed COVID-19 case, it is recommended that you arrange a COVID-19 PCR test for diagnosis.
Testing After Vaccination
After vaccination, the amount and time needed to produce antibodies may vary depending on the type of vaccine used and the immune response of the individual. We recommend conducting the INDICAID COVID-19 Rapid Antibody Test at least 2 weeks after getting your second dose of vaccine.
We recommend conducting the INDICAID COVID-19 Rapid Antibody Test at least 2 weeks after getting your second dose of vaccine. If the first test indicates a negative result, you may consider conducting a second test in 2 weeks. If the first test indicates a positive result, you can consider conducting the test monthly or as needed.
If only the control line (C) is produced, it is indicative of a negative result. This means that no COVID-19 antibodies have been detected. Since the production of antibodies may vary from person to person, you can conduct another test in 2 weeks or consult your medical provider for more information.
A positive test result indicates that the individual is currently or previously infected by SARS-CoV-2 or has developed an immune response to the SARS-CoV-2 vaccine.
IgM antibodies are the first type of antibodies produced in response to the SARS-CoV-2 antigen. IgG is only produced after a process called seroconversion which takes place 7-15 days after the onset of symptoms or post vaccination. IgM antibodies will stay in the body for 1-8 weeks and production will gradually diminish. You can consider arranging another test for IgG antibodies in 2 weeks.
If the control line (C), IgM line, and IgG line are present, it means that both the IgM and IgG antibodies are present in your body.
The color intensity of the test lines may vary. Any test line, even if faint, should be interpreted as a line and is indicative of the presence of the relevant antibody. This test only performs qualitative detection of antibodies and does not provide quantitative data on specific antibodies.
It is still possible to get infected by SARS-CoV-2 after vaccination. However, compared to people who have not been vaccinated, vaccinated individuals tend to have a lower viral count and exhibit less severe symptoms upon infection. How long antibodies last in the body may vary from the type of vaccine used and from person to person.
Product Resource
INDICAID COVID-19 Rapid Antibody Test Instruction Guide